Fertility
“We gravitate towards things that are instantly gratifying. IVF is one of them”

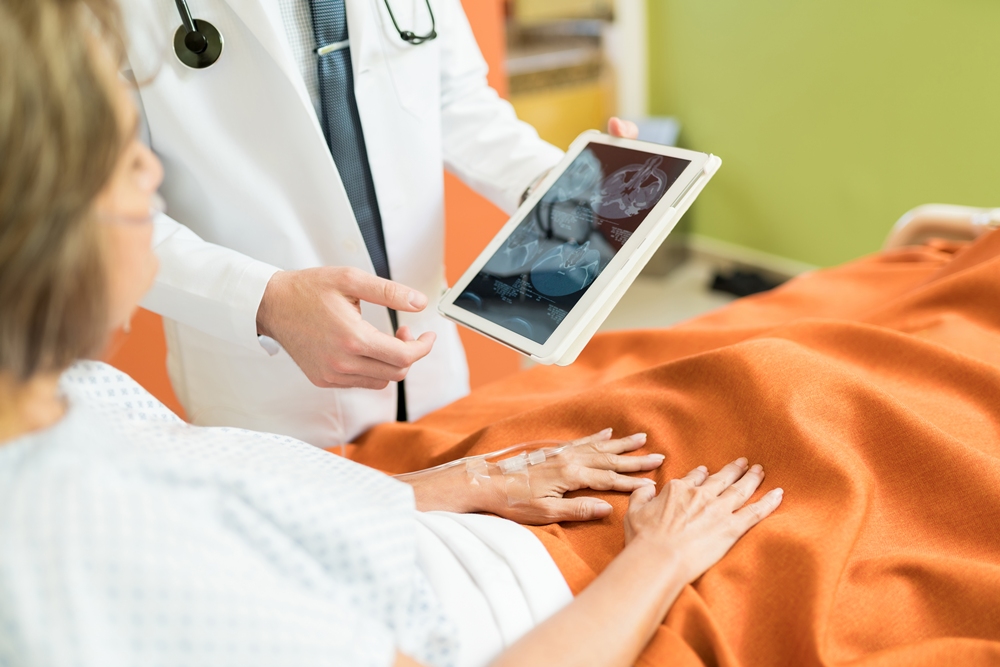
As the global IVF market is estimated to reach US$36.2b by 2026, embryologist Cynthia Hudson tells FemTech World why IVF has become the logical response when struggling to conceive.
It is estimated that infertility affects one in seven heterosexual couples in the UK.
Since the original NICE guideline on fertility published in 2004 there has been an increase in the prevalence of fertility problems and a greater proportion of people seeking treatment.
A study from 2021 published in Sociology of Health & Illness found that in the UK, IVF is presented as an entirely predictable, and linear course of action when dealing with infertility.
The increasing age at which women start families, declining male fertility along with greater social acceptance of fertility treatment has meant the market has been enjoying steady growth in recent years and so has IVF.
“If a couple runs into a perceived level of infertility, the quickest way to get pregnant statistically, is to do IVF,” says Cynthia Hudson, embryologist and VP of clinical strategy at TMRW Life Sciences.
“The reason why the odds are stacked so heavily in our favour is because we can effectively condense time. We can stimulate the ovaries to produce multiple eggs at once, as opposed to the typical menstrual cycle output of one egg per month.
“But there is a certain level of impatience in our world, and we tend to gravitate towards things that can get us to our stated goals faster. In the rush, we may not take the time to understand all of the reasons behind the infertility.”
There is a lack of awareness around what might require IVF and what might not, says Hudson. Age, lifestyle choices and timing are often overlooked, although they play an important role in achieving parenthood.
“IVF may be required as part of your fertility journey, but it’s also important to treat what the symptom is in the first place, if it is possible to do so,” Hudson explains.
“Age is the most significant predictor of success. So, if you are 25 and you’re not achieving quick success, it’s unlikely that the eggs are the cause of the problem. It could be a timing issue or an issue on the male side.
“If you’re in your 40s, however, it is likely that age is why you can’t become pregnant – your chance of success is reduced. I think it’s important to accelerate the decision-making process at this point.
“You don’t need a fertility specialist to tell you that you’re 42. But sometimes we just get impatient, and we don’t take the time to investigate the cause. We will go right to solution, rather than try to see if we can figure it out.”
During IVF, an egg is removed from the woman’s ovaries and fertilised with sperm in a laboratory. The fertilised egg or the embryo is then returned to the woman’s womb to grow and develop.
As embryos vary in quality, research has predominantly focused on advancing the methods of selecting embryos. However, scientists are now looking at ways to improve the gamete and embryo quality to begin with.
“We’ve spent a lot of time investigating how to screen the embryos and choose the best of the cohort, but what we haven’t really done is improve the egg and sperm cohorts to begin with,” says Hudson, adding that working on the development and growth of the gametes is an exciting area.
“At this point, all we can do is take the material that we have, and then put them together. But what we want to do is try and make that cohort develop better, so that we could improve success rates both in vitro (IVF) and in vivo (in the human body), and help couples conceive without IVF in the first place.”
Cellular reprogramming – the act of reverting mature, specialised cells into induced pluripotent stem cells – is another interesting area of research, says Hudson.
“This means you would get a cheek swab, you would submit that to a lab and the researchers would then deprogram those cells back to where they were at their earliest point. Then they would get those together with an egg or a sperm and eventually, grow an embryo out of that without having to go through the entire egg retrieval cycle.
“Although this is not something that we are likely to see in the next five years, it remains an area that will be further explored.”
Currently, many couples still face barriers to getting fertility treatment. According to the World Health Organisation (WHO), between 48 million couples and 186 million individuals struggle with infertility globally, but only three per cent have access to IVF and similar treatments.
Disparities – largely determined by complex social, cultural, racial, and economic factors – require further studies and cultural enrichment.
For embryologists like Hudson, improving access to fertility treatment remains a priority.
“The vast majority of humans do not have access to fertility treatment and that’s a tragedy. I think we owe it to ourselves and our fellow humans to change that.
“I would like to get to a place where we can say what definitively does or does not work in the lab to improve pregnancy rates. We need to stop charging people for treatments that haven’t been proven clinically beneficial”, the expert adds.
“There are a lot of manual steps in the IVF process right now and we’re at a point where we need to standardise these in order to help us figure out how to improve. I’ve focused a lot of my time recently trying to raise the bar and automate some of those processes.
“We need to make sure that we don’t leave things to chance. We don’t have the infrastructure yet, but we’re getting there.”
For more info about TMRW Life Sciences, visit tmrw.org.
News
Scientists turn human skin cells into eggs in IVF breakthrough

Researchers have created human eggs from skin cells, in a breakthrough that could transform IVF treatment for couples who have no other options.
The work remains at an early stage, but if scientists can refine the process it could allow women who are infertile due to age, illness or medical treatment to have genetically related eggs.
The same technique could also be used to make eggs for same-sex male couples.
Prof Shoukhrat Mitalipov, who led the research at Oregon Health and Science University in Portland, said: “The largest group of patients who might benefit would be women of advanced maternal age.
“Another group are those who have been through chemotherapy because that can affect their ability to have viable eggs.”
While women are expected to be the primary beneficiaries, the skin cells used to make eggs need not come from potential mothers.
“We used female skin cells in this study, but you could use skin cells from males as well,” Mitalipov told the Guardian.
“You could make eggs for men, and that way, of course, this would be applicable to same-sex couples.”
The work draws on cloning techniques pioneered in the 1990s at the Roslin Institute in Scotland.
A team led by the late Ian Wilmut used somatic cell nuclear transfer – a process that moves genetic material between cells – to create Dolly the sheep.
The procedure involved removing the nucleus (the cell’s control centre containing genetic information) from an adult sheep cell and placing it into a sheep egg that had had its own nucleus removed.
The resulting embryo was carried to term in a surrogate mother.
The Oregon team took a similar approach by collecting skin cells from women and removing the nucleus from each.
The nucleus, which contains 46 chromosomes carrying around 20,000 genes that make up the human genetic code, was placed into healthy donor eggs that had had their own nuclei removed.
The main challenge for scientists was that healthy human eggs normally contain only 23 chromosomes.
Another 23 come from sperm during fertilisation, producing the full set of 46 required for development into an embryo and eventually a baby.
Writing in Nature Communications, the Oregon team described how they tackled the problem of excess chromosomes.
After fertilising the eggs with sperm, they activated them using a compound called roscovitine.
This caused the eggs to move roughly half of their chromosomes into a structure called a polar body – a small cell formed during egg development – leaving the remaining chromosomes to pair with those from the sperm.
In a healthy fertilised human egg, 23 chromosomes from the mother pair with 23 from the father.
However, the Oregon team found that in their lab-created eggs, the chromosomes paired up at random. This led to embryos with the wrong number of chromosomes or incorrect pairings.
“These abnormal chromosome complements would not be expected to result in a healthy baby,” said Prof Paula Amato, a co-author of the study at Oregon.
The team is now working to refine the process.
Of the 82 eggs created, fewer than 10 per cent developed to the stage at which embryos are typically transferred during IVF.
None were cultured beyond six days, suggesting the process remains inefficient.
Mitalipov described the work as a “proof of concept” with more challenges ahead. Perfecting the method and proving its safety in patients could take another decade.
“I think it’s going to be harder than what we’ve done over the years thus far, but it’s not impossible,” he said.
Other scientists praised the breakthrough.
Prof Richard Anderson of the University of Edinburgh said: “Many women are unable to have a family because they have lost their eggs, which can occur for a range of reasons including after cancer treatment.
“The ability to generate new eggs would be a major advance.
“There will be very important safety concerns, but this study is a step toward helping many women have their own genetic children.”
Diagnosis
NHS introduces new ovarian cancer screening for high-risk women
High-risk women can now delay ovary removal surgery through NHS screening, helping them preserve fertility and avoid early surgical menopause.
The ROCA test tracks women with BRCA gene mutations every four months, monitoring cancer risk markers instead of requiring immediate preventive surgery.
University College London Hospitals NHS Foundation Trust has begun rolling out the programme, with plans for national expansion after approval from the National Institute for Health and Care Excellence (NICE).
BRCA1 and BRCA2 mutations greatly increase the risk of breast and ovarian cancer. Normally, BRCA genes repair damaged DNA, but when faulty they allow tumours to grow more easily.
Breast cancer screening with mammograms is long established, but until now there has been no equivalent for ovarian cancer. Women carrying BRCA mutations were typically advised to have their ovaries removed preventively – a step that ends fertility and triggers menopause immediately.
The ROCA test monitors changes in CA125, a blood protein that can indicate ovarian cancer when levels rise.
By testing every four months, women can keep track of their risk while delaying surgery if they wish.
Professor Adam Rosenthal, consultant gynaecologist at UCLH, said the test was designed as a damage-limitation option for women not ready for surgery.
A raised result means patients can reconsider surgery, while regular monitoring increases the chance of detecting cancer early, when outcomes are better.
Natasha Wray learned she carried the BRCA gene after breast cancer treatment at 36. She was advised to have her ovaries removed but refused.
She said: “I was 36 at the time, and I said, absolutely not. I’d just gone through seven rounds of chemo, extensive surgery, and had sort of a year and a half, two years of my life caught up in cancer treatment, and I just wanted to be left alone.
“And I also didn’t want to go through a surgical menopause in my mid-30s.
“I also very much wanted to be a mum, so I definitely wanted to hold on to any fertility that I did have.
She initially paid privately for the ROCA tests and admitted they brought anxiety.
But Rosenthal said the screening helps keep the decision about surgery at the forefront for patients.
At 41, Wray had a baby. Two years later, her test results showed raised CA125 levels, and she delayed before eventually choosing surgery.
“It wasn’t easy,” she said.
“But I do think it’s really important as well that women’s bodies aren’t just seen as baby making bodies and machines.
“I very much wanted to be a mother. That wasn’t my sole purpose for holding onto my ovaries.
“It was very personal and it actually had nothing to do with anybody else. It was very much me, how I felt in my body, what had already been felt like to me, taken from me.”
NICE has approved the ROCA test for BRCA carriers, with health officials highlighting its cost-effectiveness and aiming to make it available across England.
News
Preconception BMI linked to fertility and miscarriage risks

Preconception BMI outside the healthy range is linked to longer time to pregnancy and higher miscarriage risk, new research has revealed.
The study followed 3,033 pregnancy or preconception episodes among couples in Rotterdam between August 2017 and July 2021, examining how body mass index (BMI) – weight relative to height – affects fertility.
Women had a median age of 31.6 years and men 33.4. BMI categories were underweight (under 18.5), normal (18.5–24.9), overweight (25–29.9) and obese (30 or greater).
Fecundability – the chance of conceiving in one menstrual cycle – declined with each BMI unit for both sexes.
Women with obesity had a 28 per cent lower chance of conceiving than women of normal weight, while overweight women had a 12 per cent reduction.
Overweight and obese men had an 11 per cent reduction compared with normal-weight men.
The researchers wrote: “A better understanding of the separate and combined associations of BMI in women and men with fertility and miscarriage outcomes is needed to develop novel targeted population strategies to optimise BMI from the preconception period onward.”
Of the cases studied, 17.8 per cent of women were classed as subfertile, meaning conception took more than 12 months. In addition, 11.3 per cent of pregnancies ended in miscarriage, defined as loss before 22 weeks’ gestation.
Underweight women had nearly twice the risk of subfertility compared with normal-weight women.
Overweight women faced a 35 per cent higher risk, while women with obesity had a 67 per cent higher risk.
Miscarriage risks were also higher outside the healthy BMI range: overweight women had a 49 per cent higher risk and women with obesity a 44 per cent higher risk than normal-weight women.
The median time to pregnancy across all participants was 3.7 months. The study also recorded use of assisted reproductive technology, including in vitro fertilisation, among couples struggling to conceive.
The investigators concluded: “Optimising BMI from the preconception period onward in women and men might be an important strategy to improve fertility and pregnancy outcomes.”

 News10 hours ago
News10 hours agoMothers’, not fathers’, mental health directly linked to their children’s, study shows

 Menopause5 days ago
Menopause5 days agoNew report exposes perimenopause as biggest blind spot in women’s health

 News8 hours ago
News8 hours agoScientists turn human skin cells into eggs in IVF breakthrough

 News3 weeks ago
News3 weeks agoThe Future of femtech: Rebuilding the investment landscape

 Menopause1 day ago
Menopause1 day agoDaily pill could delay menopause ‘by years,’ study finds

 Features4 weeks ago
Features4 weeks agoUCL spin-out raises £2.5m to improve infant health with breast milk microbiome

 News4 weeks ago
News4 weeks agoWeightWatchers debuts menopause programme with Queen Latifah

 News4 weeks ago
News4 weeks agoWomen in UK with PCOS facing widespread failures in treatment, report finds




























Pingback: Exposing IVF errors key to improving standard of care, say embryologists - FemTech World